CLEVELAND — As Northeast Ohio health care facilities prepare to distribute COVID-19 vaccinations, the outline of a plan is emerging.
Officials are aiming for two goals: To save lives and slow the spread of the coronavirus, according to the Ohio Department of Health's website.
In the Cleveland area, the Cleveland Clinic and University Hospitals will both distribute the vaccine over the coming weeks, they say.
However, the number of doses that will be available and time frames for the phased rollout are still being worked through, according to the Cleveland Clinic website.
A limited number of doses of the vaccine is expected to begin arriving this week or next.
“As supply increases, COVID-19 vaccines will be available to all Ohioans who choose to be vaccinated,” the health department posted in bold letters on its website, in part, to help dispel the rumor that people will be forced to take the vaccine.
Both Pfizer-BioNTech and Moderna have vaccines that will be distributed in Ohio.
The medicine will be shipped in thermal containers packed with dry ice. The Pfizer vaccine must be stored in freezers at temperatures from -80 degrees Celcius to -60 degrees Celcius (-112 degrees Fahrenheit to -76 degrees Fahrenheit), and protected from light, said Jeffrey Rosner, the Cleveland Clinic’s executive director of pharmacy sourcing and purchasing. Moderna’s vaccine requires normal freezing temperatures.

Both companies report that in clinical trials their vaccines had nearly 95% efficacy with no serious safety concerns.
The vaccine from both companies comes in two doses, which must be given three weeks apart, and is injected into a muscle, usually the upper arm.
Distribution will be divided into phases, starting with “critical groups,” according to the Ohio Department of Health. That includes health care and front line workers, EMS responders, and staff and residents of nursing, veterans and group homes. Assisted-living facilities and psychiatric hospitals are also in the first group.
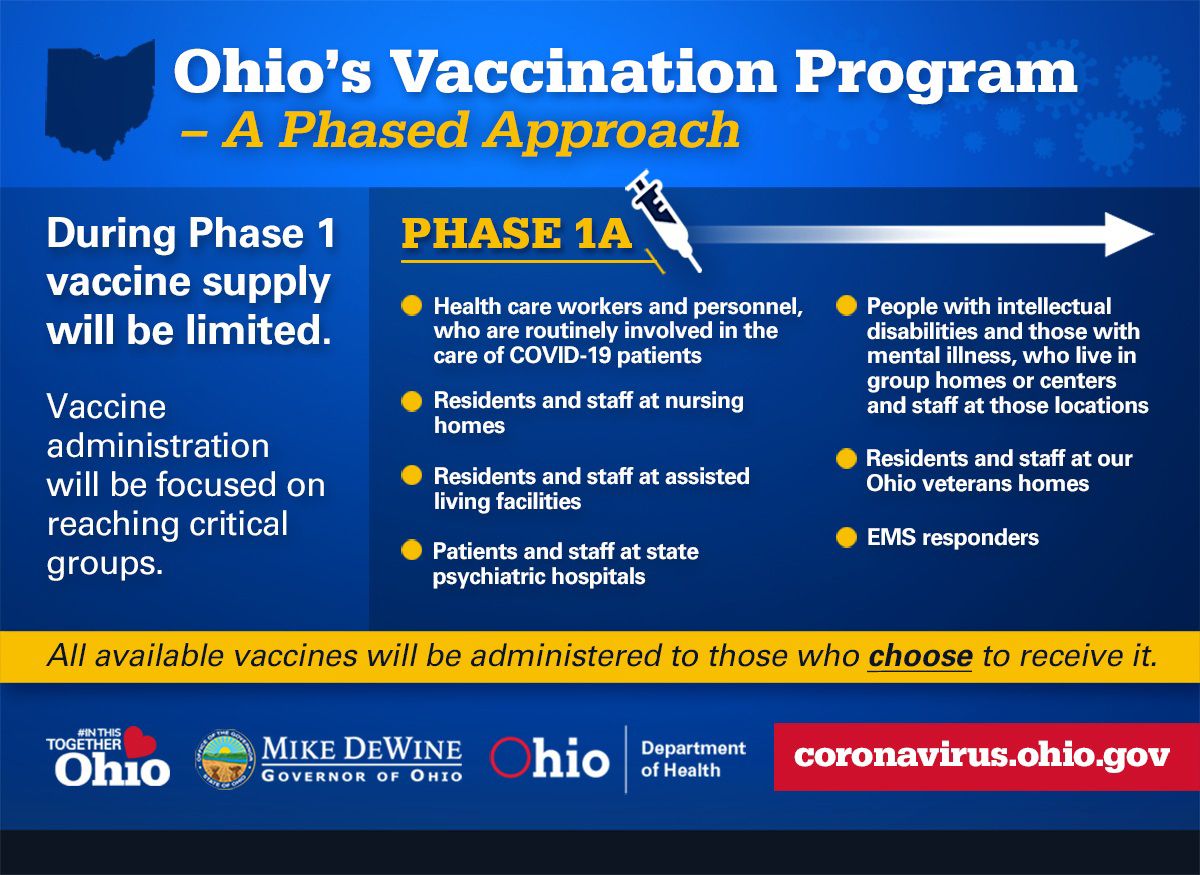
The hospitals will administer the first doses to their front-line workers, said University Hospitals COO Dr. Robyn Strosaker.
CVS and WalGreens have contracted with the government to administer the vaccine in congregate-living facilities, she said. Once those populations are inoculated, vaccines will be given to teachers, bus drivers and school staff, as well as homeless people and those incarcerated. Next are children, followed by the general public.
An unknown quantity of the Moderna vaccine is expected to arrive at University Hospitals (UH) on or near Dec. 22, Strosaker said.
With 18 hospitals in Northeast Ohio, 40 health centers and many physician practices, UH has been approved to distribute the vaccine from 13 sites, she said.
While healthcare workers will receive instructions from their employers as to when they’ll get the vaccine, she said, people not in one of the “critical groups” will likely not see a dose of the vaccine until next spring or summer.
“When there's enough vaccine distributed that we're able to get through those early phases, and we're start able to vaccinate patients, we will certainly reach out to our patients and make sure that they know exactly what they need to do,” she said.
People who receive vaccinations should not get complacent because it isn’t 100 percent effective, Strosaker said.
“It's still going to be important to wear masks, wash hands and social distance,” she said. “This is going to be important until we have enough of our communities that are needed that we really can drive down the rise of COVID. So getting the vaccine doesn't mean you can stop doing those things.”
Cleveland Clinic’s main campus near downtown Cleveland will be among the first sites to store both companies’ vaccines, as soon as they are delivered. Deemed one of Ohio’s 10 “pre-positioned” sites by the department of health, the clinic is able to distribute any vaccine that’s FDA-approved for emergency use.
The clinic established a large central storage area for the vaccine, equipped with 16 large storage freezers, with some set to ultra-cold for Pfizer’s vaccine and others set for Moderna’s temperature requirements, Rosner said in a statement.
The area is highly secured, with backup power and 24/7 temperature monitoring, he said, and additional freezers are on stand-by in case of system failure.
The Pfizer vaccine can be stored for six months if kept ultra-cold, but it is highly unstable, so care must be taken when it’s handled, Rosner said.
“Once we open up the freezer, we only have three minutes in which to pull the product out,” he said. “And then we have to close the freezer doors and the freezer doors must remain shut for two hours, so we need to pull that product quickly, get it into refrigerators and then ultimately get them off to our inoculation locations.”
The vaccine does not contain SARS-CoV-2 so it can’t give anyone who takes it COVID-19, the company reported.
But, because the vaccine was developed so quickly, people’s fears about the safety of the drug have to be addressed, said Dr. Michelle Medina, the clinic’s associate chief of clinical operations for community care, in a statement.
“I think everybody recognizes this happened in a very quick timeline. It also is on a different vaccine platform, that technology is actually the first time that we're using messenger RNA for use in vaccines in humans,” she said. “And so I think that generates a lot of anxieties. I think being very transparent about the data, being very transparent about how the data was collected, how the trials were conducted is going to be very important for the public.”
The vaccine manufacturers had to present at least two months of data for the FDA to determine they're safe, she said. There were few severe reactions, even fewer serious adverse reactions.
“ In fact, most of the reactions tend to happen fairly early, very much like other vaccines, you will have a local reaction within the first 24 or 48 hours,” she said. “They do report fevers, headaches, chills, muscle aches but I think it's also important for the public to understand that when they're experiencing that, that would be typical of vaccines that we give, even to this day.”
The clinic reports some challenges in the distribution process, such as meeting the cold-storage requirements of the vaccine, fairly distributing the “scarce vaccine supplies” and ensuring the required second doses are received.
But this is not the first full-scale vaccination program hospitals have undergone, Medina said. The H1N1 vaccination was broadly distributed as well, so health care facilities have experience.
“But there's still a lot of new things relative to the COVID vaccine that we're going to have to learn. So we ask for your patience,” she said. “We are working as hard as we can to really be on top of the science, to be on top of the logistics, to make this a very safe process for everybody who wishes to have a vaccine.”



