With the opioid epidemic still plaguing the country, a growing number of companies are submitting applications to the Food and Drug Administration hoping to get over-the-counter approval for their version of an opioid overdose reversal medication.
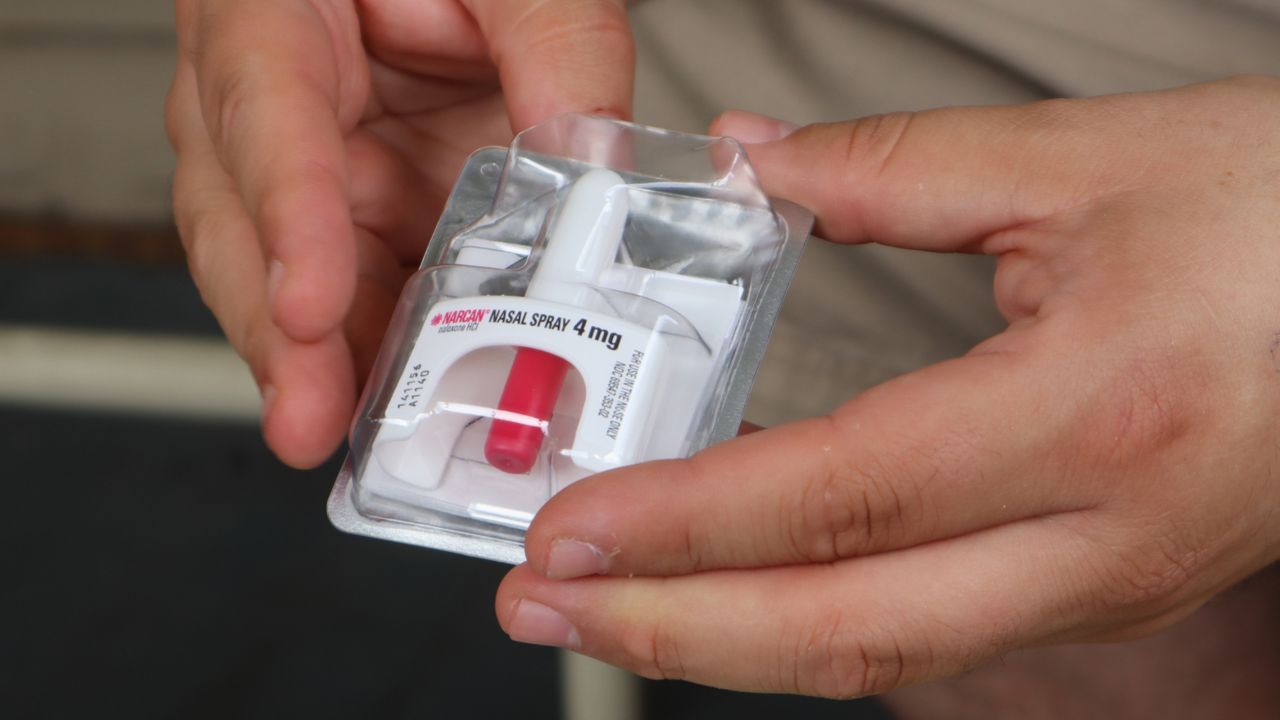
The latest such company is Emergent BioSolutions, manufacturer of the widely-used nasal spray Narcan.
Emergent BioSolutions in early December announced its nasal spray was granted priority review from the FDA to become an “over-the-counter (OTC) emergency treatment for known or suspected opioid overdose,” the first such drug to receive the fast-track review.
Priority review means the FDA aims to evaluate the submission on the shortened timeline of six months as opposed to the usual 10 months for typical review, and gives the designation to drugs that the agency says “would significantly improve the treatment, diagnosis, or prevention of serious conditions.”
Narcan, which was first approved for prescription use by the FDA in 2015, is an intranasal naloxone hydrochloride spray used in cases of suspected opioid overdoses.
Robert G. Kramer, president and CEO of Emergent BioSolutions, wrote in a statement that the company "is committed to increasing access and awareness of naloxone, and we are taking this step to help address the rising and devastating number of opioid overdoses and fatalities happening across the country."
The fast-tracked review process would likely put Emergent’s OTC approval ahead of at least one other competitor that announced plans to submit applications to the FDA and, if approved, would be the first-ever over-the-counter naloxone to go to market. Emergent anticipates the FDA’s review to be completed by March 29, 2023, though that date is not set in stone.
Pocket Naloxone Corp., a start-up company that aims to increase widespread access to the overdose reversal drug, in early December announced results from a study of its naloxone nasal swab indicating it takes effect more quickly than the nasal spray and intramuscular options on the market.
The NaxSwab device, which Pocket Naloxone Corp. CEO and co-founder Ashanthi Mathai said was “under final stages of development” at the start of the month, was designed as an easy-to-use product that Mathai hopes will gain over-the-counter approval from the FDA at some point in the near future.
"We are committed to making it affordable and conveniently purchasable in retail stores and online as an OTC product,” Mathai wrote in a statement. According to The Wall Street Journal, Pocket Naloxone plans to apply for new drug approval early next year, and will request priority approval.
The swath of applications comes soon after the FDA in November issued a notice encouraging companies to apply for over-the-counter approval for their naloxone products, saying that a preliminary assessment found certain products might “be approvable as safe and effective for nonprescription use.”
“Today’s action supports our efforts to combat the opioid overdose crisis by helping expand access to naloxone,” FDA commissioner Robert M. Califf wrote in a statement. “The agency will keep overdose prevention and reduction in substance use disorders as a key priority and area of intense strategic focus for action as rapidly as possible.”
In order to expand over-the-counter access to the overdose reversal drug, the agency said it requires additional information from companies including “product-specific data on the nonprescription user interface design, including packaging and labeling,” and more.
The notice also only applied to certain naloxone products, namely nasal sprays with a dose of up to four milligrams and intramuscular or subcutaneous injections with a dose of up to two milligrams.
The opioid epidemic has become an increasing problem in the United States over the past several decades, after prescribers began issuing pain management prescriptions for legal opioids like oxycodone, hydrocodone, morphine and more in the late 1990s. In 2010, overdose deaths from heroin began to increase dramatically, and in 2013 overdose deaths from synthetic opioids – namely, fentanyl – began to spike, according to the Centers for Disease Control and Prevention.
Opioids are currently the main driver of overdose deaths in the United States, with a little over 82% of opioid-related overdose deaths involving synthetic opioids.
Approximately 75,673 died from opioid-related overdoses in the 12 months before April 2021, up from 56,064 in the same time period a year prior.
In a call with reporters last week, director of the White House Office of National Drug Control Policy Dr. Rahul Gupta said the U.S. has seen over 81,00 opioid-related deaths over the past 12 months, adding that “experiencing a nonfatal overdose is one of the most important predictors of a future fatal overdose.”