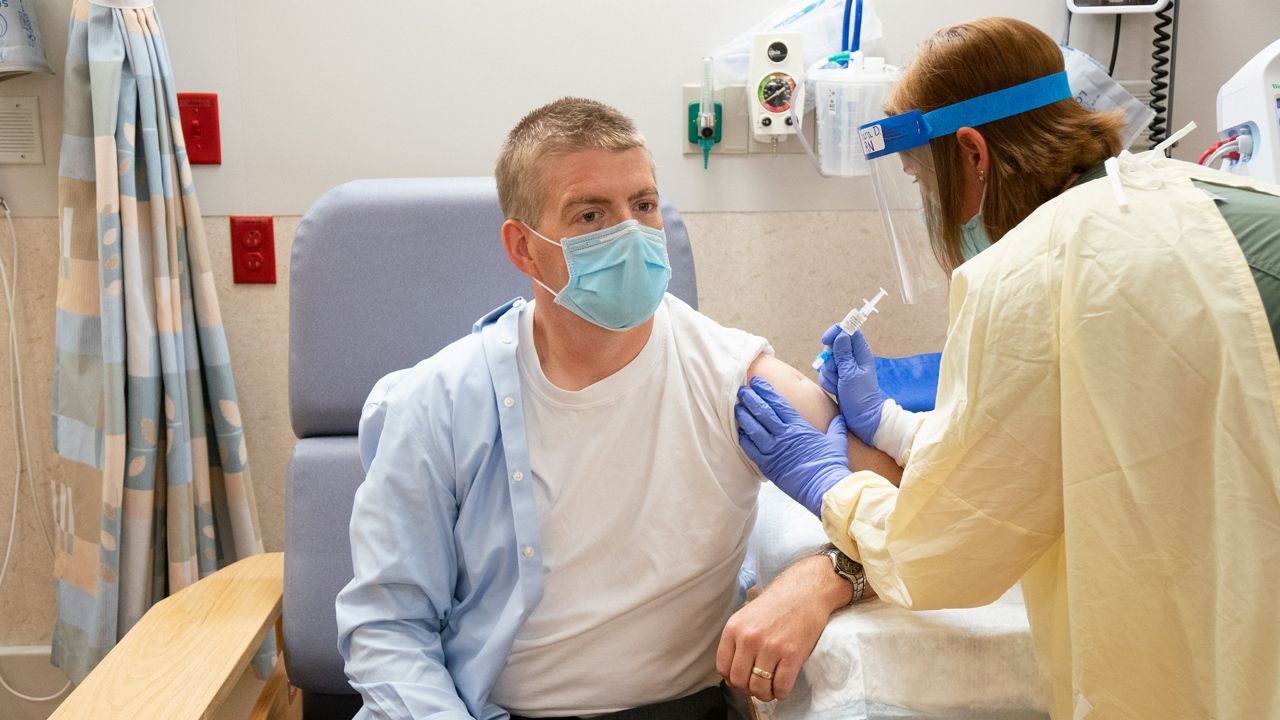
MADISON, Wis. (SPECTRUM NEWS) — The race for a vaccine that can stop COVID-19 in its tracks has rallied scientists from across the world into action. That includes researchers at UW Health, who are busy testing out the candidate vaccine developed by Oxford University and AstraZeneca.
Two other companies released promising results for their own vaccine trials this week: Moderna announced its preliminary data showed a 94.5% efficacy rate, and Pfizer, whose latest results found a similar 95% rate, said Friday it was seeking authorization from the FDA.
There’s still a ways to go before the Oxford-AstraZeneca trial can release its own efficacy results, says William Hartman, the principal investigator for the UW Health trials. But he’s already “very excited” about the promising results in the worldwide vaccine race.
“These are game changers,” Hartman says. “This means that if we're able to vaccinate a sufficient number of people throughout the world, we can get to a point of herd immunity and hopefully eradicate this virus from the Earth.”
On Thursday, Oxford-AstraZeneca researchers did publish some results from phase two trials, showing that the vaccines appeared safe and induced an immune response.
But the phase three efficacy trials — which test how well the vaccine protects people from getting sick — aren’t ready to release results yet. These were put on hold Sept. 6 after an unexplained illness was reported in one U.K. participant.
Though the participant made a full recovery, Hartman says the FDA still had to complete a full investigation — looking at that patient’s records as well as safety data for the whole trial. Even as the U.K. trials resumed in under a week, the U.S. research was left on pause for around a month and a half, getting the green light to start back up again on Oct. 23.
“We have the most stringent safety standards in the world,” Hartman says. “There’s lots of thresholds that have to be met, and the FDA has not cut any corners. They've ensured the public and they've ensured us that when a vaccine comes available, it's going to be safe and effective.”
Since restarting, Hartman says the study was up to around 150 enrolled participants in Wisconsin this week, and they’re adding dozens more each day with the goal of bringing on between 800 and 1,000.
When there are enough participants enrolled, and enough of them are infected with COVID-19 while going about their lives, researchers will be able to dig through the data to figure out how effective the shots have been. Oxford professor Andrew Pollard, the chief investigator for the AstraZeneca trials, told the BBC this week that he expected some results would be available “before Christmas.”
Like in the other companies’ trials, the AstraZeneca study includes one group of patients who receive the actual experimental vaccine and another control group that receives a placebo injection. Because the study is double-blind, the patients don’t know which shot they’re getting — and neither do the researchers, Hartman explains.
“Once the enrollment is completed, there has to be a certain number of COVID cases that happen within the population,” he says. “Once they hit that number, they're able to unblind those individuals to see who developed COVID in the vaccine group versus the placebo group.”
In the Pfizer trial, out of 170 total participants who caught COVID-19, only eight had gotten the vaccine injection — the rest were all in the control group. Moderna’s interim results similarly showed that only five of the 95 COVID-19 cases reported had gotten the vaccine rather than the placebo.
These two vaccines do have a different setup from the AstraZeneca vaccine, Hartman says, but all have the same essential goal: Getting your cells to produce the coronavirus’s signature spike protein. This way, the body learns to recognize the virus and produces antibodies to fight it off, so your immune system is primed in case you do encounter the virus.
“What's encouraging is that, even though they're different vaccines, the end point is really the same,” Hartman says. “Provided that we're all making the same antibodies to those spike proteins, we would expect the results on the vaccines to be quite similar.”
Pfizer and Moderna’s versions use messenger RNA, a novel approach that delivers genetic instructions to cells on how to produce the spike protein. The AstraZeneca vaccine is instead made from what’s called a chimp adenovirus vector, or a weakened version of a common cold virus, Hartman explains.
Scientists “take out the part that would have made the chimp sick, and they insert the part that makes that spike protein from the coronavirus,” he says. Then, when the vaccine gets injected into a person’s shoulder, it is able to invade cells and use their machinery to produce the spike protein, which builds up and spurs the immune system into action.
Even as Pfizer and Moderna appear closer to getting authorization, the AstraZeneca vaccine, if effective, could still be important for getting more people immunized. Because one thing is becoming increasingly clear in this vaccine race: There probably won’t be just one winner.
“It’s going to take multiple vaccines to really vaccinate the entire population of the world, or as much of it as we can,” Hartman says.
As more information comes out, we may learn that certain vaccines are better suited for different populations, he points out. Plus, the mRNA vaccines need to be frozen, while other versions — including AstraZeneca’s — don’t require the same level of cold storage, which may make them easier to transport.
At a briefing on Tuesday, officials from the Wisconsin Department of Health Services said they were already taking into account the complicated logistics of distributing different COVID-19 vaccines.
“[COVID-19 vaccine distribution] poses significant challenges, such as the need for ultracold storage and multiple vaccines from multiple manufacturers on different schedules,” Deputy Secretary Julie Willems Van Dijk said. “However, this is what public health care's up for.”
So, while the recent results have been promising, the work of scientists, manufacturers, and public health officials is far from over. And until a vaccine can be widely distributed, protective measures like staying home and wearing masks are essential to curbing the sky-high coronavirus rates we’re seeing across the country.
In the meantime, Hartman says he’s excited to be playing a role in solving the problem that has mobilized top scientists all across the world.
“The amount of time and effort that takes to put together these trials … it's been unbelievable,” Hartman says. “And to be part of that effort to fight this pandemic that has really paralyzed our society and societies around the world — to try to bring that to an end — it's humbling, and it's awesome at the same time.”