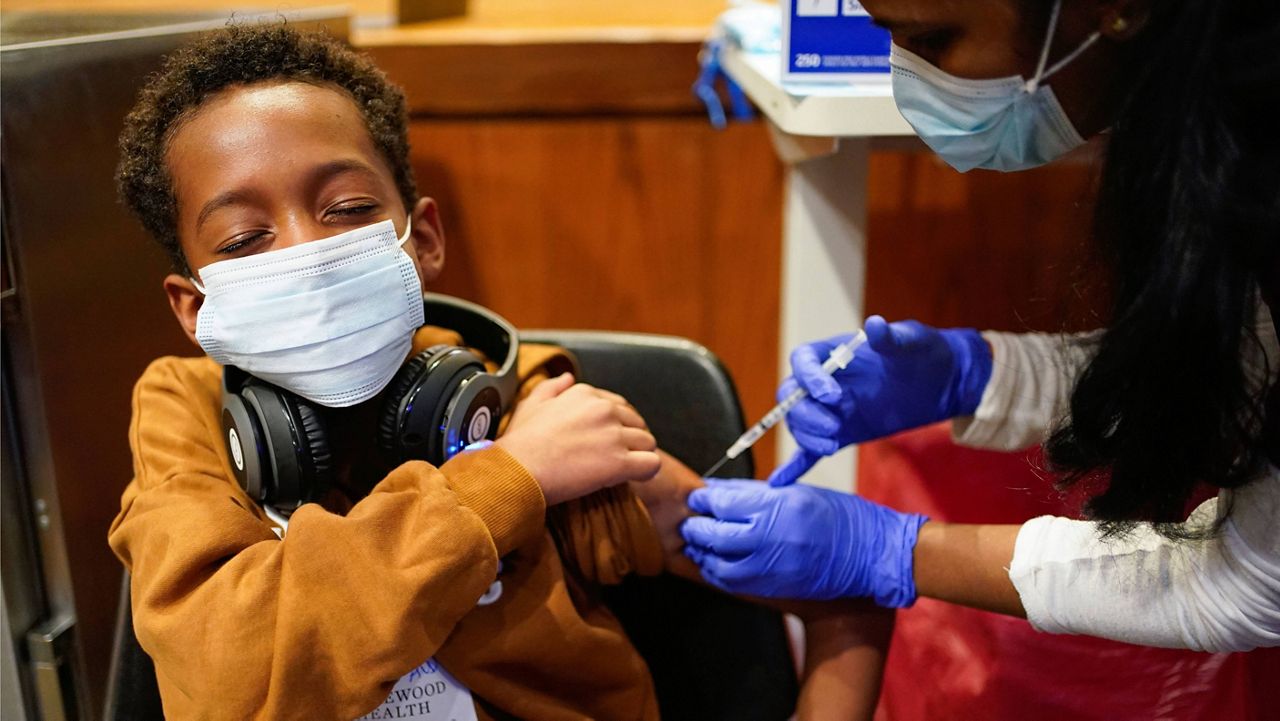
More than half of parents surveyed in a new poll expressed doubts about the safety and effectiveness of the COVID-19 vaccine for children, a sign that inoculating an overwhelming majority of kids could be a struggle.
What You Need To Know
- More than half of parents surveyed expressed doubts about the safety and effectiveness of the COVID-19 vaccine for children, a sign that inoculating an overwhelming majority of kids could be a struggle.
- According to a new ABC News-Washington Post poll, just 46% of adults with a child younger than 18 at home said they’re confident the vaccine is safe for 5- to 17-year-olds
- Forty-seven percent said they are confident the shot is effective at preventing serious COVID-19 in that age group
- A clinical trial found the Pfizer-BioNTech shot to be safe and nearly 91% effective at preventing symptomatic infections in elementary school-age children
According to a new ABC News-Washington Post poll, just 46% of adults with a child younger than 18 at home said they’re confident the vaccine is safe for 5- to 17-year-olds. Forty-seven percent said they are confident the shot is effective at preventing serious COVID-19 in that age group.
Fifty-two percent said they are not so or not at all confident about the vaccine’s safety and effectiveness.
The numbers were starkly different when the same poll asked adults about COVID-19 vaccines in September. In that survey — in which participants were asked about vaccines generally, not specifically for children — 71% called the shots safe, and 72% said they believed they are effective.
Skepticism about the children’s vaccine’s safety and effectiveness is higher among Republicans and Republican-leaning independents, the survey found. Mothers are more likely than fathers to trust the vaccine is effective, and members of minority groups are more likely than white parents to believe the shot is safe, according to the poll.
The poll surveyed 1,001 people by phone between Nov. 7-10.
The Food and Drug Administration granted emergency use authorization to the Pfizer-BioNTech vaccine for children ages 5 to 11 last month, and the Centers for Disease Control and Prevention recommend the shots for those kids Nov. 2, making 28 million more people eligible for vaccination.
A clinical trial found the shot to be safe and nearly 91% effective at preventing symptomatic infections in elementary school-age children.
The Pfizer-BioNTech vaccine has been available to 12- to 17-year-olds since May.
According to The Washington Post, 46% of 12- to 17-year-olds have been fully vaccinated. Federal health officials said last Wednesday that more than 900,000 children 5 to 11 had received their first dose and that at least 700,000 others had scheduled their shots.