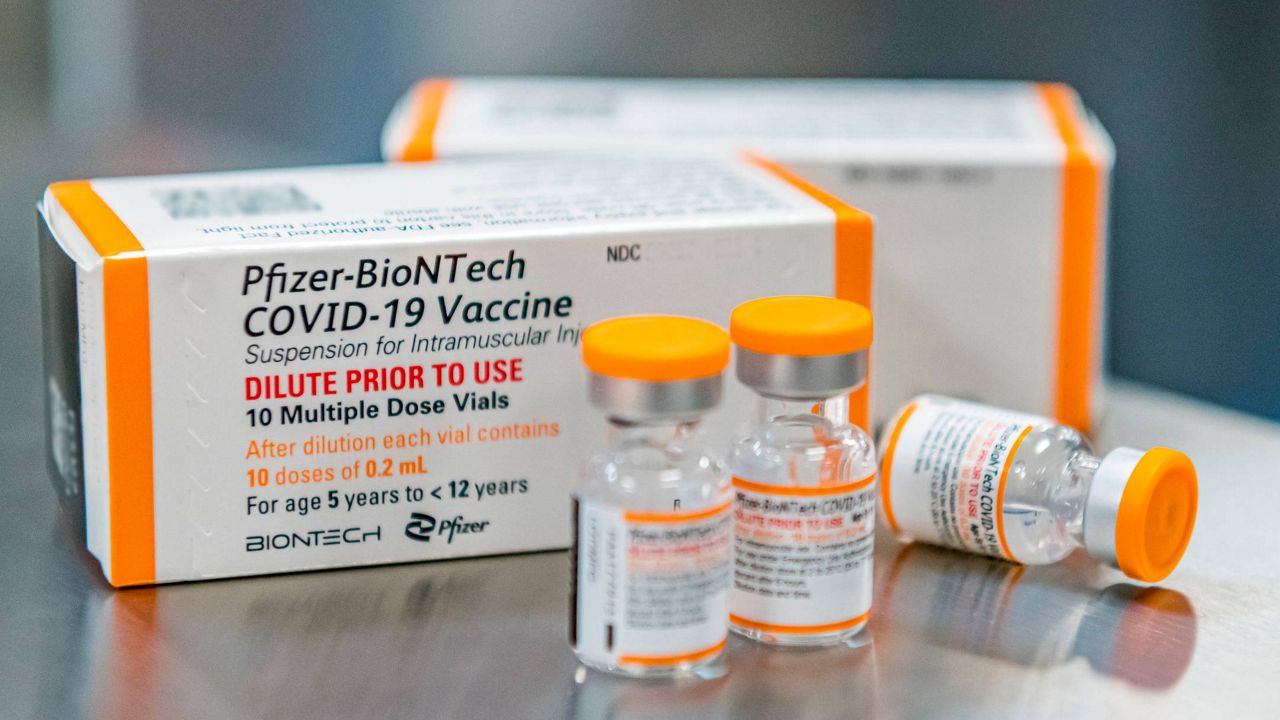The wait for children to get vaccinated against COVID-19 is over.
The director of the Centers for Disease Control and Prevention (CDC) has given the final clearance for children 5-11 to receive doses of the Pfizer-BioNTech COVID-19 vaccine, clearing the way for shots to begin "as soon as possible."
CDC Director Dr. Rochelle Walensky accepted the recommendation of an influential advisory panel to the agency, which unanimously endorsed vaccines for the 5-11 age group earlier Tuesday.
"CDC now expands vaccine recommendations to about 28 million children in the United States in this age group and allows providers to begin vaccinating them as soon as possible," the agency wrote in a statement.
Shortly after the decision, Hartford HealthCare in Connecticut posted a video to Facebook showing some of the first young children to receive the vaccine.
The move follows the Food and Drug Administration (FDA) authorizing smaller-size doses for the younger age group last week. The Pfizer shots for kids use a lower dosage — a third of what is given to adults.
The shots are the first to be made available in the United States to children younger than 12 and make an additional 28 million Americans eligible for vaccination. Millions of doses have already been shipped to states nationwide in advance of the CDC's decision.
In a statement, President Joe Biden called the decision "a turning point" in the nation's fight against COVID-19.
"It will allow parents to end months of anxious worrying about their kids, and reduce the extent to which children spread the virus to others," the president wrote. "It is a major step forward for our nation in our fight to defeat the virus."
"A vaccine for children age 5 to 11 will allow us to build on the extraordinary progress we’ve made over the last nine months," Biden continued. "Already, more than 78 percent of Americans age 12 and older have gotten at least one shot, including millions of teenagers — and the vaccines have proven to be incredibly safe and effective."
"Today is a monumental day in the course of this pandemic," Walensky told the influential Advisory Committee on Immunization Practices (ACIP) earlier Tuesday. "There are children in the second grade who have never experienced a normal school year. Pediatric vaccination has the power to help us change all of that."
Pfizer’s clinical trial found the two-dose shot to be nearly 91% effective at preventing symptomatic infection in 5- to 11-year-olds. The shots are administered three weeks apart. FDA scientists reviewed and affirmed Pfizer’s findings.
Pfizer found no new or unexpected side effects. Those that did occur mostly consisted of sore arms, fever or achiness.
However, FDA scientists noted that the study wasn't large enough to detect extremely rare side effects, including myocarditis, a type of heart inflammation that occasionally occurs after the second dose.
In their analysis, FDA scientists concluded that in almost every scenario the vaccine's benefit for preventing hospitalizations and death from COVID-19 would outweigh any serious potential side effects in children.
Children are at a lower risk than adults of developing severe COVID-19, but serious cases do occur, and youths can spread the virus to others.
Nearly 2 million children 5 to 11 have been infected with the virus, more than 8,300 have been hospitalized, and nearly 100 have died. Coronavirus hospitalizations among children under 18 surged this summer before hitting their highest point of the pandemic in early September.
And COVID-19 has been blamed for more than 2,500 cases of multisystem inflammatory syndrome, which causes inflammation of the heart, lungs, kidneys, brain, skin, eyes or gastrointestinal organs. The virus also can cause myocarditis.
Federal officials said Monday that the vaccination program for kids should be "fully up and running" the week of Nov. 8.
"Over the last several weeks, my Administration has been working hard to be prepared for this moment: we are ready to act," Biden wrote in a statement Tuesday. "We have already secured enough vaccine supply for every child in America, and over the past weekend, we began the process of packing and shipping out millions of pediatric vaccine doses. These doses — specially designed for these younger children — have started to arrive at thousands of locations across the country."
"The program will ramp up over the coming days, and fully up and running during the week of November 8," Biden continued. "Parents will be able to bring their children to thousands of pharmacies, pediatrician’s offices, schools, and other sites to get vaccinated.
"Because of the groundwork we’ve laid, we can be confident that vaccinations for kids will be available, easy, and convenient," the president added.
The federal COVID-19 response team has been “preparing for weeks,” coordinator Jeff Zients in a briefing Monday, including by securing enough supply for 28 million children in recent weeks.
“Starting the week of Nov. 8, the kids’ vaccination program will be fully up and running,” Zients said Monday. “Parents will be able to schedule appointments at convenient sites they know and trust to get their kids vaccinated, and the number of sites will continue to increase throughout the month.”
“I deeply understand the urgency and concern over providing the best protection to our children against the virus,” Dr. Walensky said Monday.
Walensky outlined the data around the Pfizer vaccine for young kids, including the fact that it can be up to 91% effective in preventing COVID-19 and studies found no serious side effects among children who got the shot.
“We also know parents will have a lot of questions. And I would encourage parents to ask questions as they consider the benefits of vaccinating their children,” Dr. Walensky added, noting that it’s also critical to surround kids with vaccinated adults for their protection.