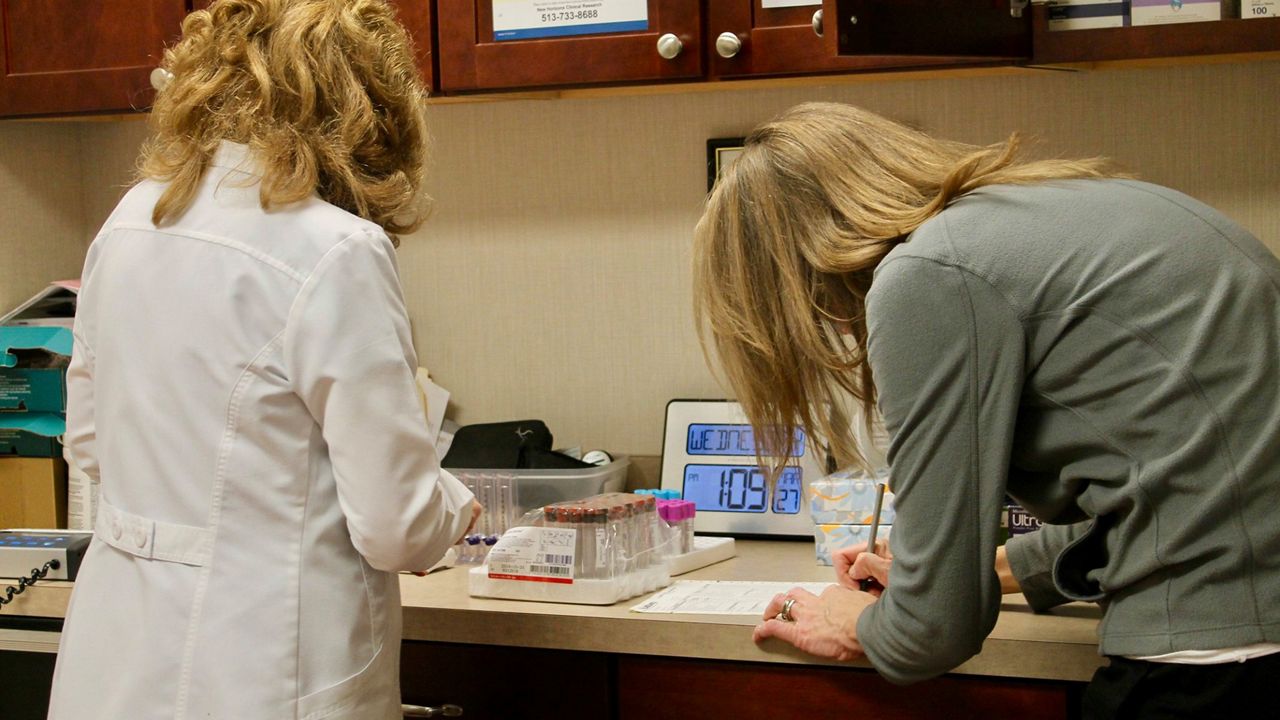
COLUMBUS, Ohio — A clinical research company enrolled hundreds of adolescents in the Moderna vaccine trial at its site in Blue Ash, Ohio, the company’s president said.
What You Need To Know
- Moderna has requested emergency use authorization for individuals 12 and older
- The vaccine was tested in adolescents at labs including a site in Blue Ash, Ohio
- Velocity Clinical Research enrolled hundreds of Ohio participants in the Moderna trial
Paul Evans of Velocity Clinical Research said the Hamilton County site registered 300 volunteers for the trial, which had a total of 3,732 participants in the 12 to 17 age group.
Moderna submitted analysis of the trial results to the Food and Drug Administration in a June 10 request for emergency use authorization for the adolescent population.
Expanded authorization of the Moderna vaccine would give teenagers a second highly effective option in addition to the Pfizer-BioNTech vaccine, which was approved in May for individuals 12 and older, Evans said in a Zoom interview.
“I've been doing clinical research for over 30 years now — and that study went as well as just about any that I’ve ever come across,” Evans said.
Finding participants for COVID-19 trials has been easier than any previous vaccine trials Velocity has done because their calls for participants have been quickly met with volunteers eager to participate.
“The under-18 age group that came forward for the study knew an awful lot about the study. They were very well-informed and were very motivated to take part and be part of the solution for the COVID-19 pandemic,” he said.
The strong interest level among adolescents allowed Velocity to complete recruiting in four to six weeks. That step in a vaccine trial would traditionally take six to nine months, he said. It’s one of the reasons Evans said the COVID-19 vaccine timeline could be expedited without cutting corners.
Overall, Velocity has enrolled more than 10,000 people in various COVID-19 vaccine trials. In Cleveland, the company also enrolled participants in the Novavax vaccine trial for adolescents, which recently completed registration, as well as the Pfizer vaccine trial.
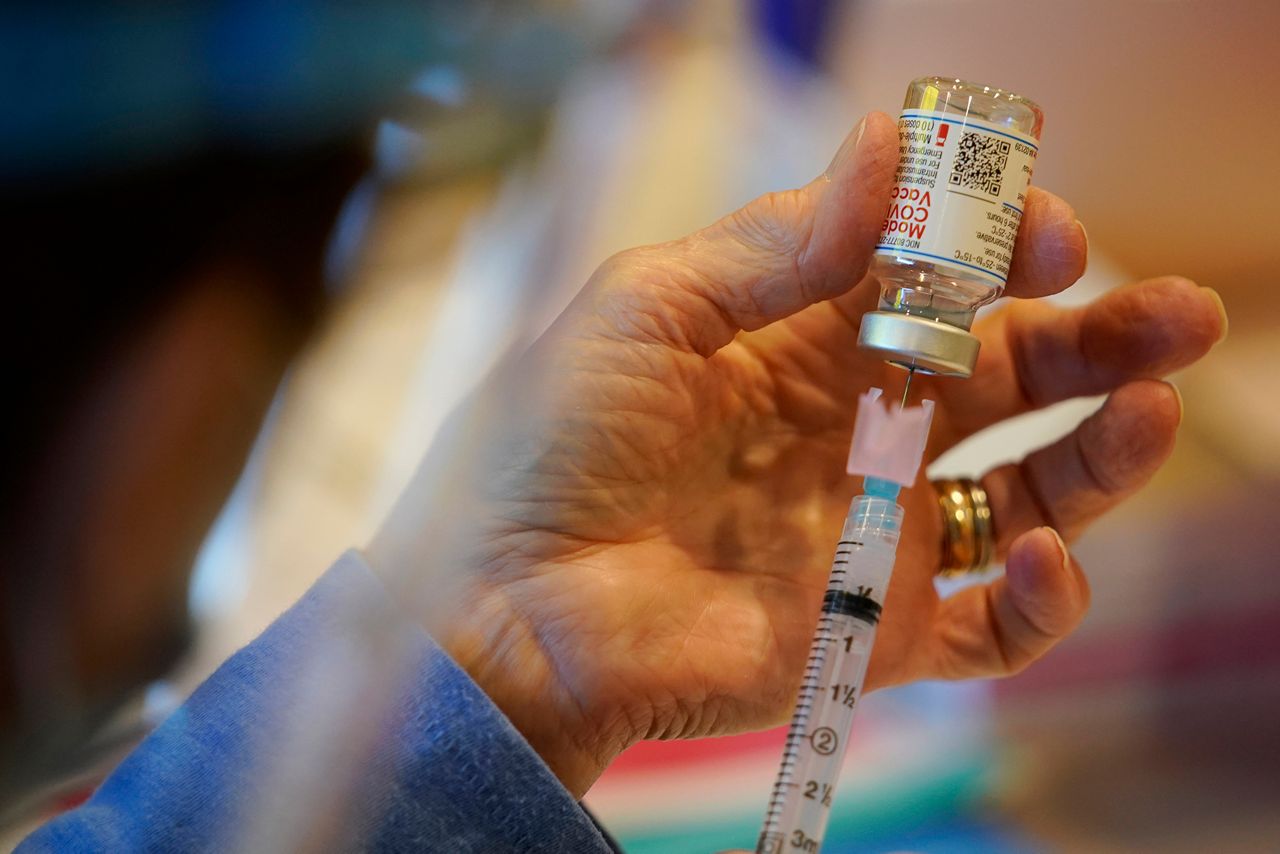
Velocity said the adolecent trial has gone smoothly.
According to Evans, the research thus far is showing the mRNA vaccines have strong safety profiles across the age group.
“The adolescent groups are tolerating the vaccine better even than the adult groups,” he said.
The volunteers in the adolescent Moderna trial will be monitored for two years. The drug manufacturer said the results so far demonstrate that the vaccine is effective preventing COVID-19 and generally well tolerated among adolescents, according to the request for authorization.
Moderna has begun the next phase of vaccine study — enrolling children in the 5-11 age group. Velocity is participating in the pediatric study. While they have not enrolled Ohio volunteers yet, Evans said he expects that will happen soon, but the trial is following a process starting the enrollment in phases to ensure there is a diverse mix of ages represented in the study.