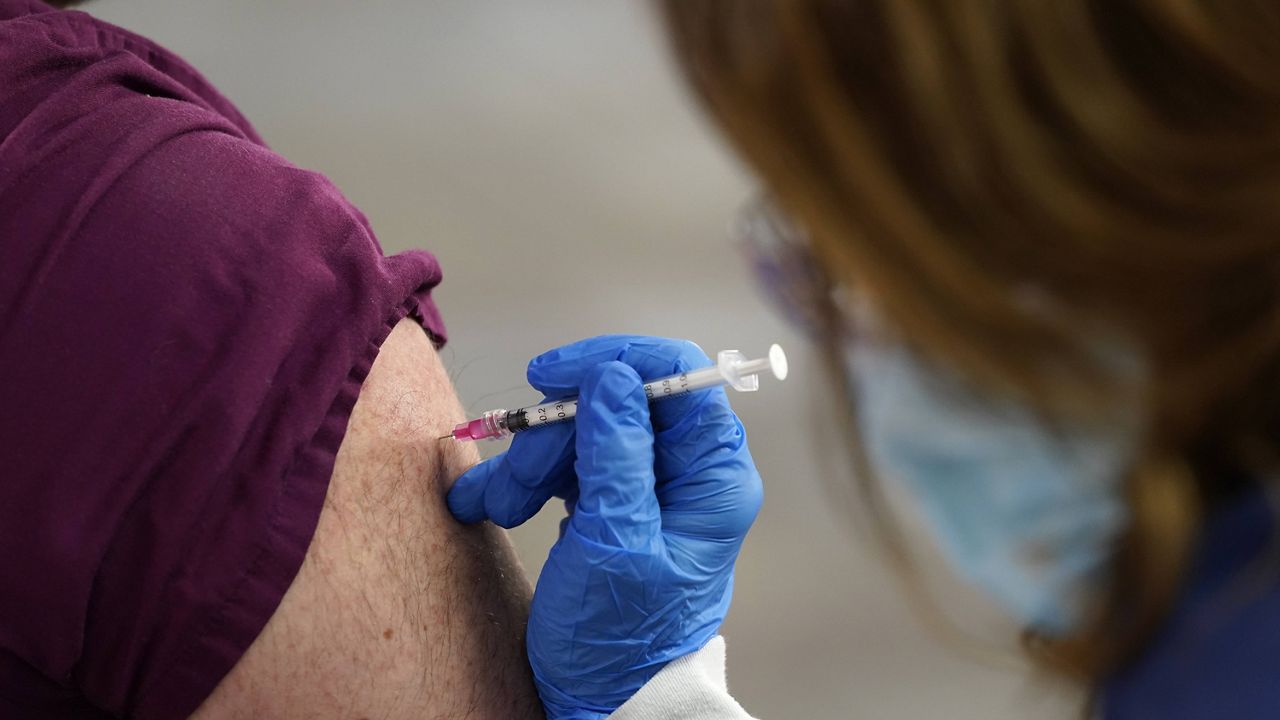
The Food and Drug Administration is warning health care workers not to deviate from the authorized coronavirus vaccine schedules and dosages.
What You Need To Know
- The Food and Drug Administration is warning health care workers not to deviate from the authorized coronavirus vaccine schedules and dosages
- In recent days, calls have grown for alternative ways to vaccinate more Americans sooner, including adminsitering half doses as well as doses reserved for booster shots
- FDA officials Stephen Hahn and Peter Marks released a statement saying there is no clinical trial data showing those changes would be effective
- Hahn and Marks also said those changes could place "public health at risk, undermining the historic vaccination efforts to protect the population from COVID-19"
In recent days, calls have grown for alternative ways to vaccinate more Americans sooner, fueled in part by practices being seen in the United Kingdom.
There, health officials are administering the first dose of vaccines to as many people as possible, as opposed to withholding second doses like the United States is doing. As a result, the wait for a second dose in Britain could take up to 12 weeks, rather than the three to four weeks recommended by vaccine makers.
And in special circumstances, Britain is allowing the mixing of various coronavirus vaccines given to an individual.
In the U.S. on Sunday, Dr. Moncef Slaoui, chief science adviser to the Trump administration’s Operation Warp Speed vaccine initiative, told CBS News’ “Face the Nation” that OWS was in talks with the FDA and Moderna, the company that developed one of the vaccines, to administer half-doses to people 18 to 55 years old so that more individuals could be inoculated sooner.
Slaoui claimed a half-dose “induces identical immune response” to a full dose in those age groups.
But FDA Commissioner Stephen Hahn and Peter Marks, director of the agency’s Center for Biologics Evaluation and Research, released a joint statement Monday night saying there is no data to support such changes would be effective and that they could place “public health at risk.”
“We have been following the discussions and news reports about reducing the number of doses, extending the length of time between doses, changing the dose (half-dose), or mixing and matching vaccines in order to immunize more people against COVID-19,” the FDA officials wrote. “These are all reasonable questions to consider and evaluate in clinical trials. However, at this time, suggesting changes to the FDA-authorized dosing or schedules of these vaccines is premature and not rooted solidly in the available evidence. Without appropriate data supporting such changes in vaccine administration, we run a significant risk of placing public health at risk, undermining the historic vaccination efforts to protect the population from COVID-19.”
The New York Times reported Tuesday that scientists with the National Institutes of Health, Moderna and Operation Warp Speed are analyzing research data to determine if cutting doses in half would help alleviate vaccine shortages.
The two vaccines being administered in the U.S. now — one developed by Pfizer and the other by Moderna — require booster shots 21 and 28 days later, respectively. Clinical studies show the vaccines are 94%-95% effective after two doses.
Hahn and Marks said some people have misinterpreted data from the pharmaceutical companies’ studies regarding people who did not receive a second shot at the recommended time. The FDA said those people were only tracked for a short time, “such that we cannot conclude anything definitive about the depth or duration of protection after a single dose of vaccine.”
“Using a single dose regimen and/or administering less than the dose studied in the clinical trials without understanding the nature of the depth and duration of protection that it provides is concerning, as there is some indication that the depth of the immune response is associated with the duration of protection provided,” the FDA officials said. “If people do not truly know how protective a vaccine is, there is the potential for harm because they may assume that they are fully protected when they are not, and accordingly, alter their behavior to take unnecessary risks.
“Until vaccine manufacturers have data and science supporting a change, we continue to strongly recommend that health care providers follow the FDA-authorized dosing schedule for each COVID-19 vaccine.
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, told NBC’s “Today” show last week that the U.S. had been considering scrapping its strategy of withholding second doses of vaccines so that more people could receive the first round of shots sooner.
Fauci did not outright dismiss the idea, but sounded skeptical about it himself.
“I still think, if done properly, you can do a single dose, reserve doses for the second dose and still get the job done," he said. "But there’s a lot of discussion about whether or not you want to spread out the initial vaccination by getting more people vaccinated on the first round. You could debate either way on that."
Slaoui dismissed that possibility during his "Face the Nation" interview.
“I think it's not reasonable when vaccines have been developed with two doses given 21 days apart, or 28 days given apart, and where we have the data on their safety and their efficacy and we have ... no data after one dose,” he said.
The federal government has delivered more than 15.4 million doses of vaccines to states but only 4.6 million people have been inoculated, according to the Centers for Disease Control and Prevention. The Trump administration had projected that 20 million Americans would be vaccinated by the end of 2020.