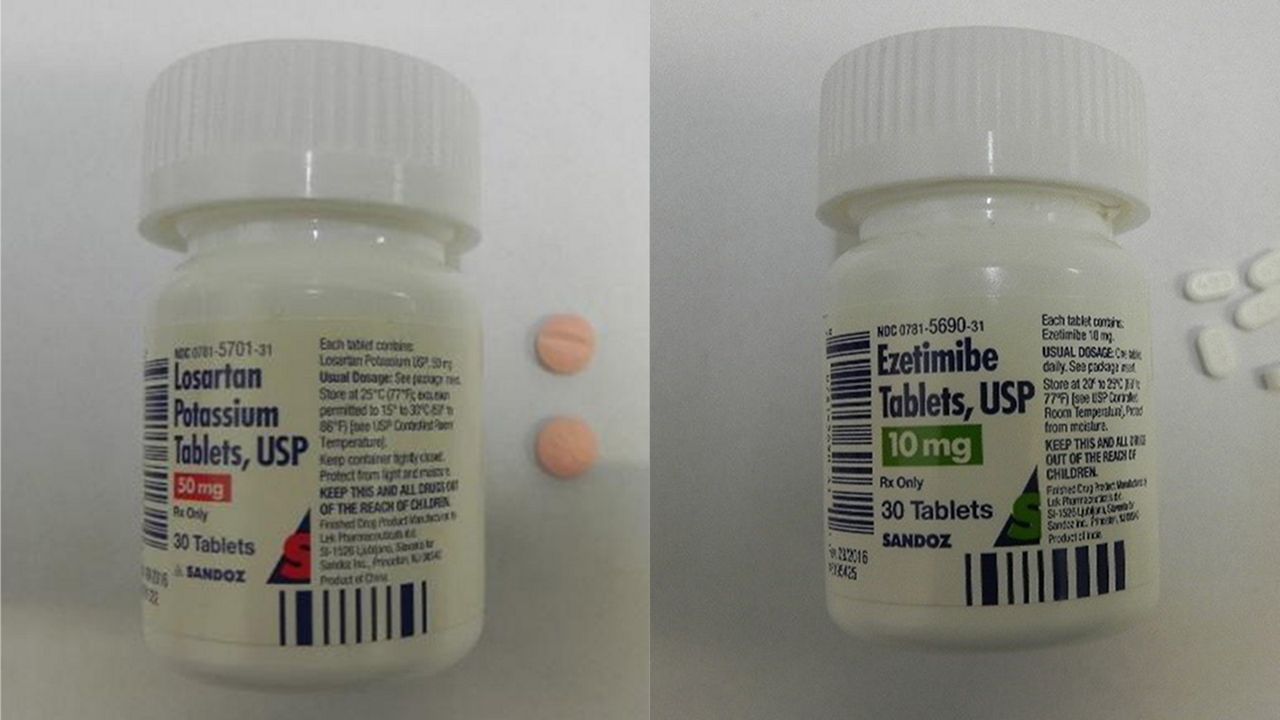
WASHINGTON, DC - The Consumer Product Safety Commission is recalling a high blood pressure and a high cholesterol medication because the bottles they are packaged in aren't child-proof.
Sandoz the maker of Ezetimibe and Losarten Potassium is recalling 600 thousand units of the medications. The Poison Prevention Packaging Act requires the medications to be child-proof, because they pose a poisoning risk if swallowed by children. The medications were sold at clinics and pharamacies nationwide from July 2018 through August 2019.
The recalled bottles feature the folloiwng on the front bottle labels
- Sandoz
- Name of the medication
- Dosage
- NDC
The lot number and the expiration date are located on the side of hte bottle labels. There is a lengthy list of lot numbers and the CPSC has them listed.
If you have the recalled medication, get it out of the reach of children and contact Sandoz for a free replacement child resistant bottle cap. There is nothing wrong with the medication, just the bottle.