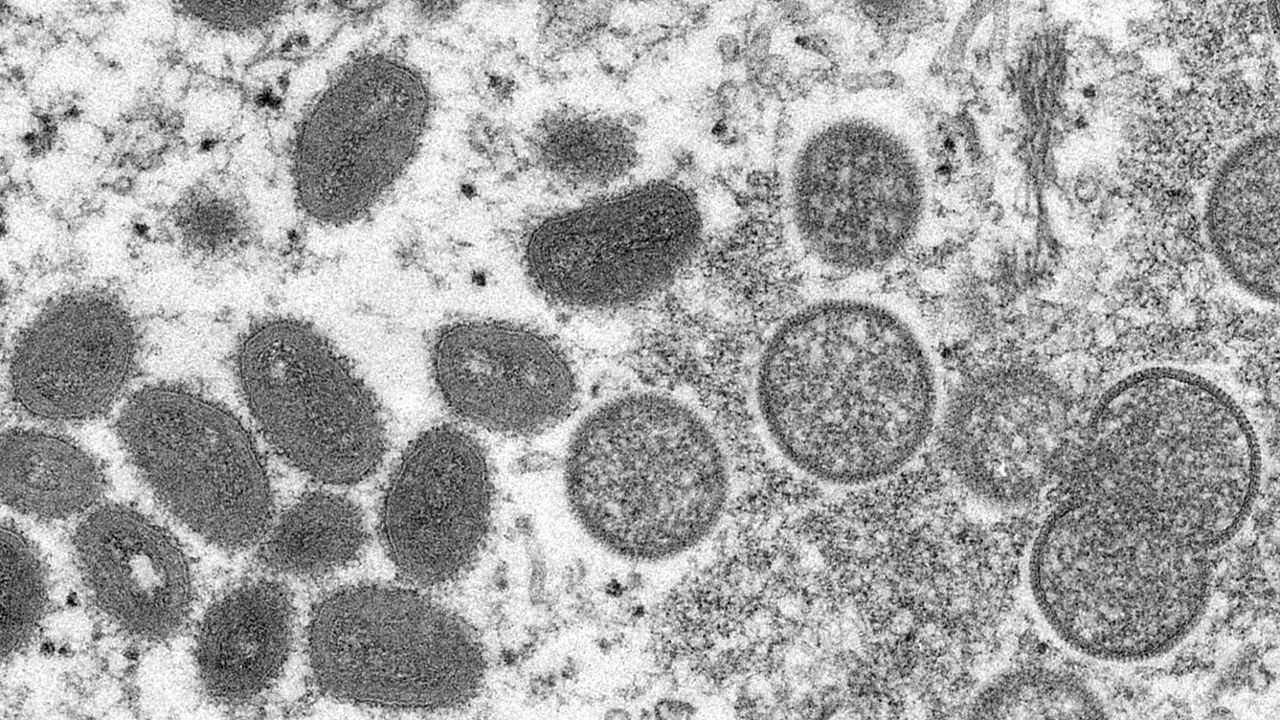
As a monkeypox outbreak draws global concern, the Centers for Disease Control and Prevention released a report Friday explaining its advisory panel’s recommendation that people with occupational exposure to orthopoxviruses be given a new vaccine.
What You Need To Know
- As a monkeypox outbreak draws global concern, the Centers for Disease Control and Prevention released a report Friday explaining its advisory panel’s recommendation that people with occupational exposure to orthopoxviruses be given a new vaccine
- The CDC, however, stressed the timing of the report was merely opportune and that the guidance was not a hasty response to the growing number of monkeypox cases, including nine in the United States
- The agency said its experts began working to refine the recommendations two years ago and the Advisory Committee on Immunization Practices, or ACIP, approved them in late 2021
- The guidance revolves around Jyennos, which in 2019 became the second vaccine authorized by the Food and Drug Administration for orthopoxviruses such as monkeypox and small pox
The CDC, however, stressed the timing of the report was merely opportune and that the guidance was not a hasty response to the growing number of monkeypox cases, including nine in the United States.
The agency said its experts began working to refine the recommendations two years ago and the Advisory Committee on Immunization Practices, or ACIP, approved them in late 2021.
The guidance revolves around Jyennos, which in 2019 became the second vaccine authorized by the Food and Drug Administration for orthopoxviruses such as monkeypox and small pox. Since 2015, the ACIP has recommended the ACAM2000 vaccine for people with occupational exposure.
ACAM2000 is a replication-competent virus that can have serious side effects such myopericarditis, a form of heart inflammation. It is a derivative of Dryvax, the vaccine that successfully eradicated smallpox by 1980.
Children in the United States received the smallpox vaccine until 1971, but a small number of people at occupational risk have continued to receive orthopoxvirus vaccines.
Jyennos is a replication-deficient vaccine. ACIP Orthopoxvirus Work Group believes, based on clinical trial data, that Jyennos is slightly more effective than ACAM2000 and less likely to result in serious side effects.
The ACIP has recommended Jyennos for lab workers doing research or testing to diagnose orthopoxviruses, certain people responding to orthopoxvirus-related public health investigations, and health care workers caring for infected patients or administering the ACAM2000 vaccine.
The committee, however, still continues to also recommend ACAM2000.
The ACIP also recommends Jyennos boosters every two years for those working with more virulent orthopoxviruses and at least every 10 years for less virulent viruses. The recommended booster interval for ACAM2000 is three years for less virulent viruses and at least 10 years for more virulent ones.
People who were given ACAM2000 for primary vaccination may take a booster dose of Jyennos, the committee’s guidance says.
Jyennos is administered as two shots given 28 days apart. ACAM2000 is given using a multiple-puncture technique through 15 jabs with a stainless steel bifurcated needle dipped into the reconstituted vaccine
Monkeypox is a virus that originates in wild animals like rodents and primates, and occasionally jumps to people. Most human cases have been in central and west Africa, where the disease is endemic.
Monkeypox begins with fever, headache, muscle aches and exhaustion followed by lesions, starting on the face and then spreading to other parts of the body.
The World Health Organization said Friday nearly 200 cases of monkeypox have been reported in more than 20 countries not usually known to have outbreaks of the disease. The agency described the epidemic as “containable.”
On Wednesday, CDC Director Dr. Rochelle Walensky said the agency has identified nine cases across California, Florida, Massachusetts, Utah, Virginia and Washington, with an additional presumed case in New York.
Ryan Chatelain - Digital Media Producer
Ryan Chatelain is a national news digital content producer for Spectrum News and is based in New York City. He has previously covered both news and sports for WFAN Sports Radio, CBS New York, Newsday, amNewYork and The Courier in his home state of Louisiana.