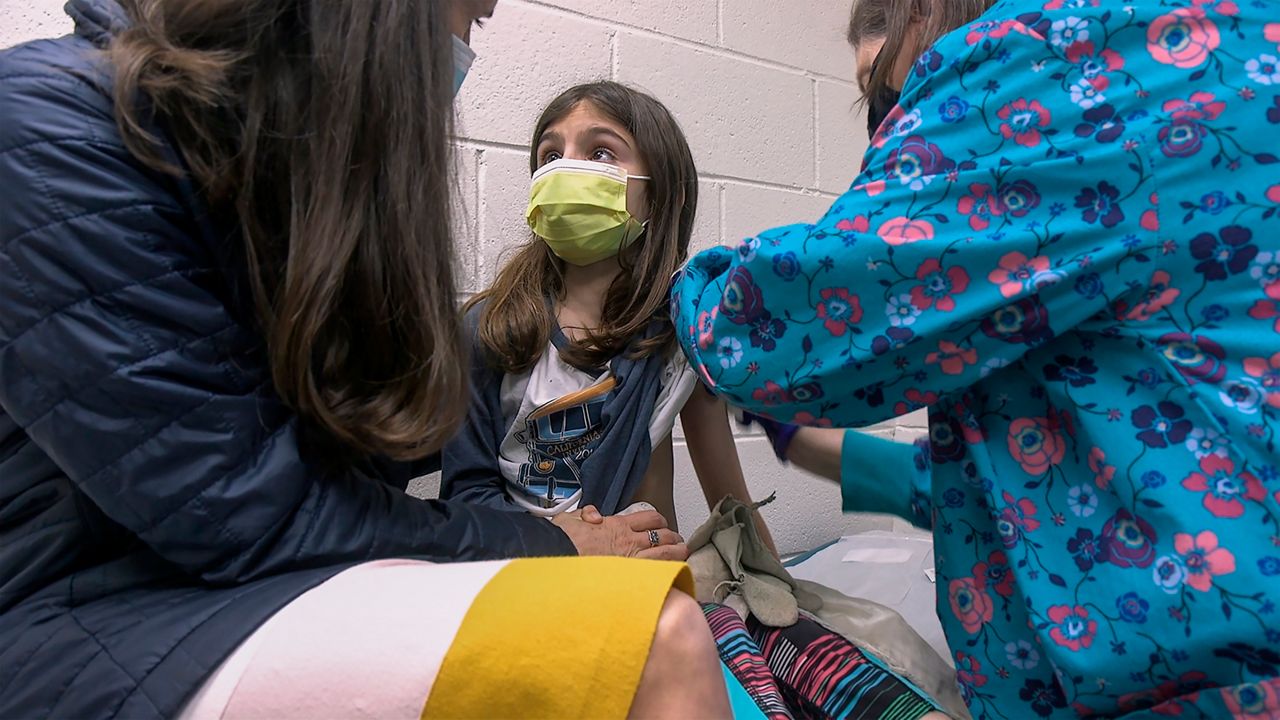
The wait for a COVID-19 vaccine for elementary school-aged children is almost over: On Friday, the Food and Drug Administration (FDA) granted emergency use authorization to Pfizer’s shots for children ages 5 to 11.
What You Need To Know
- The Food and Drug Administration granted emergency use authorization Friday to the Pfizer-BioNTech COVID-19 vaccine for children ages 5 to 11
- The move comes after the agency’s committee of outside advisers voted 17-0, with one abstention, Monday after hearing presentations from Pfizer, the FDA and the Centers for Disease Control and Prevention on the drugmaker’s trial data
- Before the shots can be made available to the public, the CDC must weigh in — its Advisory Committee on Immunization Practice will meet Tuesday
- Pfizer’s clinical trial found the two-dose shot to be nearly 91% effective at preventing symptomatic infection in 5- to 11-year-olds
The move comes after the agency’s committee of outside advisers voted 17-0, with one abstention, Monday after hearing presentations from Pfizer, the FDA and the Centers for Disease Control and Prevention (CDC) on the drugmaker’s trial data. The FDA was not required to follow the panel’s recommendation.
Before the shots can be made available to the public, the CDC must weigh in. Its Advisory Committee on Immunization Practice will meet Tuesday to discuss the vaccine and vote on whether to recommend children receive them. The CDC itself would then make the final decision on whether to issue a recommendation.
The shots would be the first to be made available in the United States to children younger than 12 and make an additional 28 million Americans eligible for vaccination.
Pfizer’s clinical trial found the two-dose shot to be nearly 91% effective at preventing symptomatic infection in 5- to 11-year-olds. The shots are administered three weeks apart.
FDA scientists reviewed and affirmed Pfizer’s findings.
Pfizer found no new or unexpected side effects. Those that did occur mostly consisted of sore arms, fever or achiness.
However, FDA scientists noted that the study wasn't large enough to detect extremely rare side effects, including myocarditis, a type of heart inflammation that occasionally occurs after the second dose.
In their analysis, FDA scientists concluded that in almost every scenario the vaccine's benefit for preventing hospitalizations and death from COVID-19 would outweigh any serious potential side effects in children.
Children are at a lower risk than adults of developing severe COVID-19, but serious cases do occur, and youths can spread the virus to others.
Nearly 2 million children 5 to 11 have been infected with the virus, more than 8,300 have been hospitalized, and nearly 100 have died. Coronavirus hospitalizations among children under 18 surged this summer before hitting their highest point of the pandemic in early September.
And COVID-19 has been blamed for more than 2,500 cases of multisystem inflammatory syndrome, which causes inflammation of the heart, lungs, kidneys, brain, skin, eyes or gastrointestinal organs. The virus also can cause myocarditis.
“The need for a safe and effective vaccine for children 5 to less than 12 years of age is clear," Dr. William Gruber, Pfizer’s senior vice president for vaccine clinical research and development, said during Monday's FDA committee meeting.
The Pfizer shots use a lower dosage — a third of what is given to adults.
The Biden administration said last week it has secured enough shots to vaccinate all kids 5 to 11 and has been working to roll the shots out quickly should regulators authorize them.
The federal government is planning to make the vaccine available at tens of thousands of pharmacies, as well as doctors’ offices, children’s hospitals, community health centers, rural health clinics and some schools.
“We will ensure that vaccinations for kids ages 5 to 11 are easy, convenient and accessible to every family,” Jeff Zients, the White House’s COVID-19 response coordinator, said earlier this month.
While green-lighting a kids shot could help boost the country’s vaccination rate of 57.6%, only about a third (34%) of parents in a Kaiser Family Foundation poll last month said they would vaccinate their 5- to 11-year-olds “right away.” Thirty-two percent said the would “wait and see” the vaccine works first, and 24% said definitely won’t get their children inoculated.
Pfizer’s vaccine is fully approved in the U.S. for people ages 18 and older, and it has emergency use authorization for 12- to 17-year-olds.
Moderna announced last week that clinical trial data show its two-dose COVID-19 vaccine generates a strong immune response in children ages 6 to 11. The company plans to submit its data, which has not yet been peer reviewed, to regulators worldwide.
Pfizer and Moderna also are testing vaccines for children as young as 6 months old. Johnson & Johnson is, too, developing vaccines for children.
The Associated Press contributed to this report.