Did you ever wonder how hot the sand is at the beach in SoCal during the peak of summer? I can't tell you how many times I wish I had my flip-flops, socks or even shoes to get from the strand or parking lot to my spot at the beach.
Doesn't it seem like the longer distance from strand to water, the hotter the sand is? Is it my imagination or is the sand actually hotter if the beach is bigger? I set out to find out the answers to these questions.
It also gave me a good reason to get out of the office. I also dragged my kid with me to get her away from her phone!

So, a couple of questions come to mind. First, why does the sand seem to get so hot–so hot that sometimes you feel like you are actually burning your feet to the point of blistering?
Also, does darker sand get hotter than lighter sand, and why? All of these questions come down to few things: the sand's albedo, specific heat and the depth of sand.
We can compare this to water. Why doesn't water get as hot as the sand? Wouldn't that be nice if SoCal beach water was warmer than its usual 50s, 60s, and–at its warmest–70s.
Right now our ocean water temperatures are in the low-to-mid-60s. That's chilly! But it sure does feel nice on your tootsies after making the 50 to 75-yard walk from the strand to the water, or to your towel and umbrella.
By the way, a longer or wider beach does not equate to hotter sand. It just feels that way because you have been on the sand longer since the distance is longer. So, by the end of your trot across the beach back to the parking lot, the sand just feels really hot!
I set out with my daughter, camera and thermometers to see if we could figure out the answers to these questions. We went to the beach at 1:30 p.m., which was about 30 minutes past solar noon (12:59 p.m. that day). We chose this time because we figured this would be when the sand would be hottest, give or take a few degrees.
Solar noon is when the sun is highest in the sky and when its rays are most direct and intense. The air temperature was 71 degrees, according to the nearest Citizen Weather Reporting station in Manhattan Beach. This recording station was one and a half blocks from the beach.
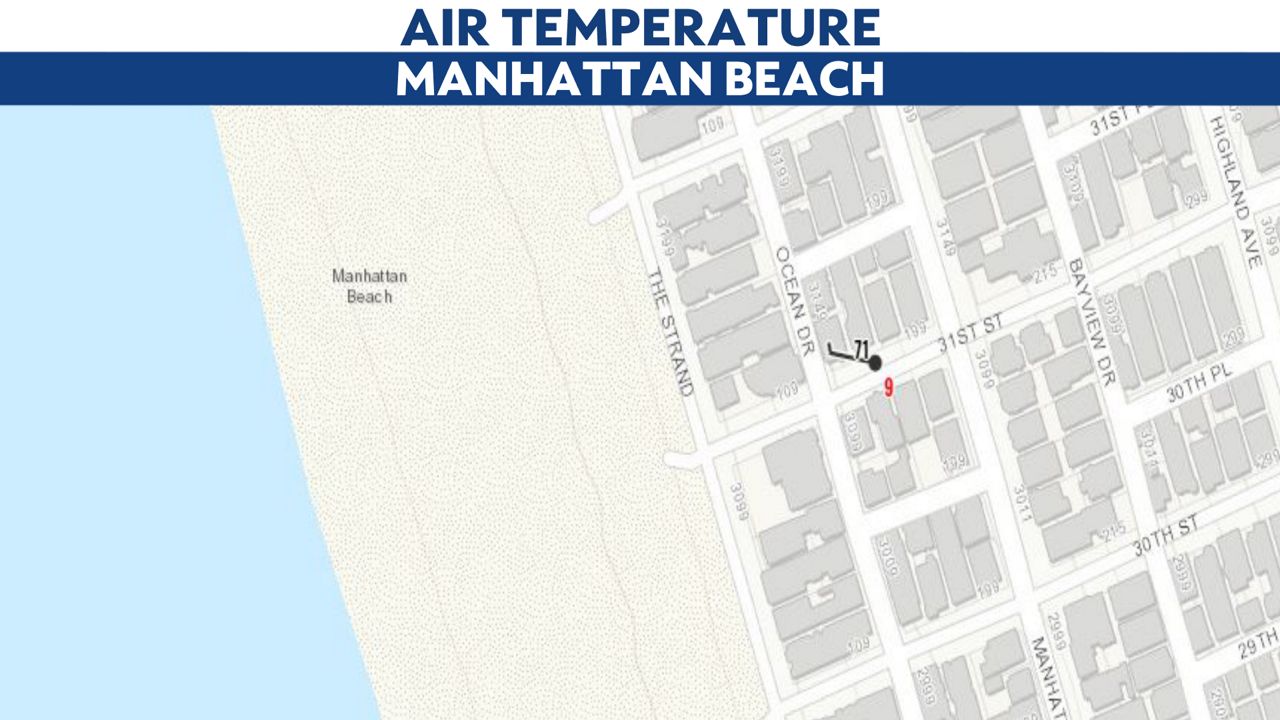
The water temperature was 65 degrees, according to the nearest lifeguard tower. You might think that the air temperature in the middle of summer in Los Angeles would be warmer.
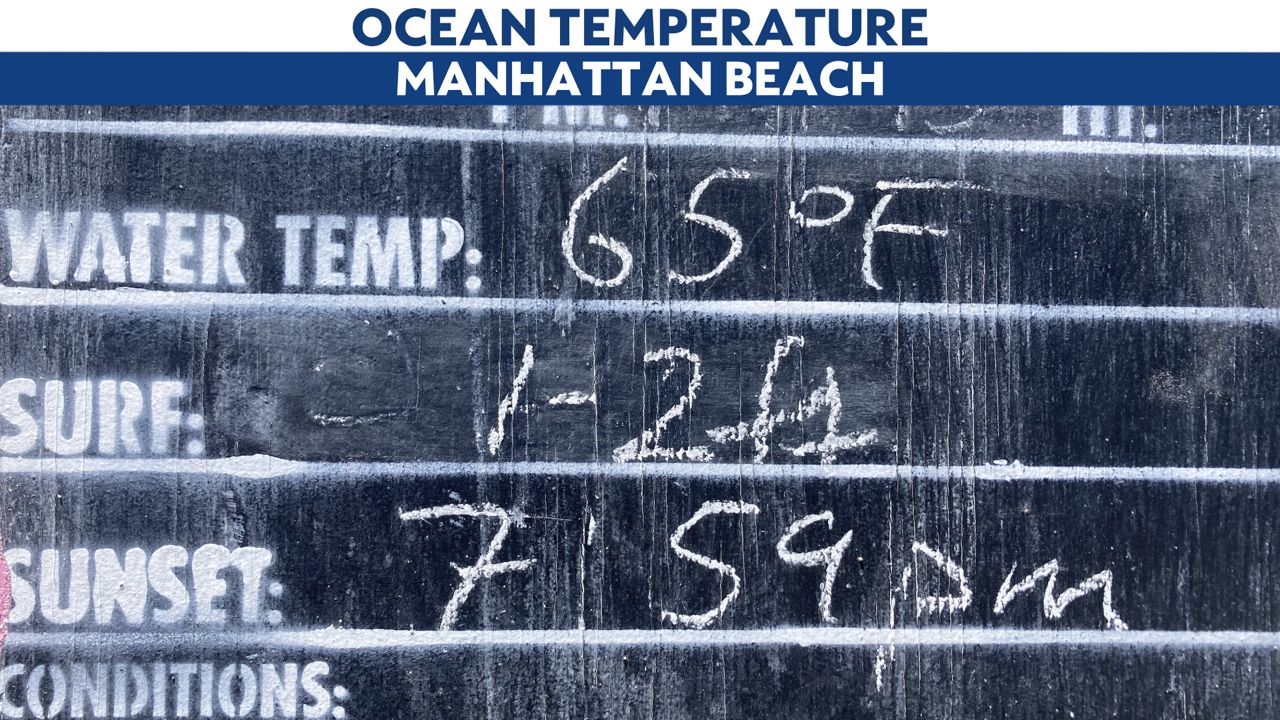
But it isn't! The air temperature is largely a function of ocean temperature.
First, how hot is the sand? I took my kitchen thermometer and laid it down within the top layer of sand. Can you guess how hot the sand was?
It was a whopping 140 degrees!

I didn't really believe it, so I took out my infrared health thermometer. Yep, 140 degrees.
Also, take note that there are a lot of black or dark grains in the sand.

No wonder it feels like my feet are actually blistering. So, how could the ocean temperature be 65 degrees, air temperature 71 degrees and the sand 140 degrees?
It comes back to those terms I touched on earlier: albedo and specific heat.
Albedo refers to the reflectiveness of a surface. A dark surface like asphalt has a low albedo and absorbs a lot of the sun's energy.
A light-colored surface like snow has a high albedo and reflects a lot of the sun's energy. Southern California beaches have a lot of dark grains, so they absorb a lot of the sun's energy. This is why the sand gets so hot.
Well, then, the ocean is dark, too. Why doesn't it heat up like the sand? This has to do with specific heat capacity. Specific heat refers to the amount of heat needed to raise the temperature of one gram of a substance by one degree Celsius.
Water has a high specific heat, while sand has a low specific heat. This means that it takes a lot more energy to raise the temperature of water compared to the amount of energy it takes to raise the temperature of sand.
Also, the sun's energy only heats the top few millimeters of sand, while the sun's energy heats the top few feet of ocean water. That's another reason the sand is so much hotter than ocean water.
Below is a chart of different substances and their specific heat in Joules. Joules are a unit of energy.
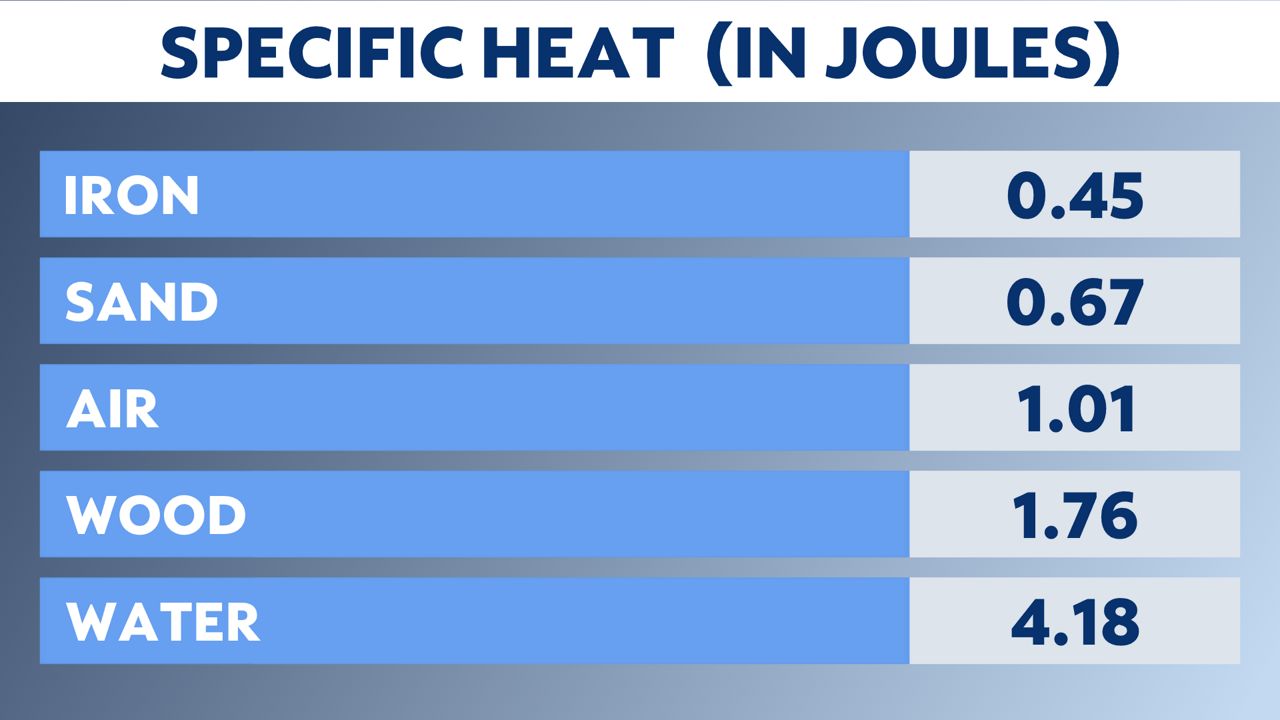
In the chart above, notice that sand is high on the list, meaning it doesn't take a lot of energy to heat it, or it heats up quickly. Water is on the bottom of the chart; it takes a lot of energy to heat. (Also, notice wood is near the bottom. That is why pot handles are made of wood.)
So, when the forecast calls for low 70s at the beach, why does it feel so much hotter, as you break out in a sweat while you are sitting in beach chair or laying down on a towel?
This is because proper placement of a thermometer on a weather station should be four to six feet above the ground in the shade with good ventilation. When you are at the beach in the early afternoon in the middle of summer, you are in the most direct and intense sun without a breeze, sitting inches from 140-degree sand.
According to broadcasters at the Tokyo Olympic Games, the air temperature at the beach volleyball court during the United States vs. Canada game was 86 degrees. The sand temperature was 108 degrees. It would have been hotter, but crews cooled the sand with water.
"Times of London" reported some practice rounds had to be canceled because players were complaining the sand was too painful to play on!



