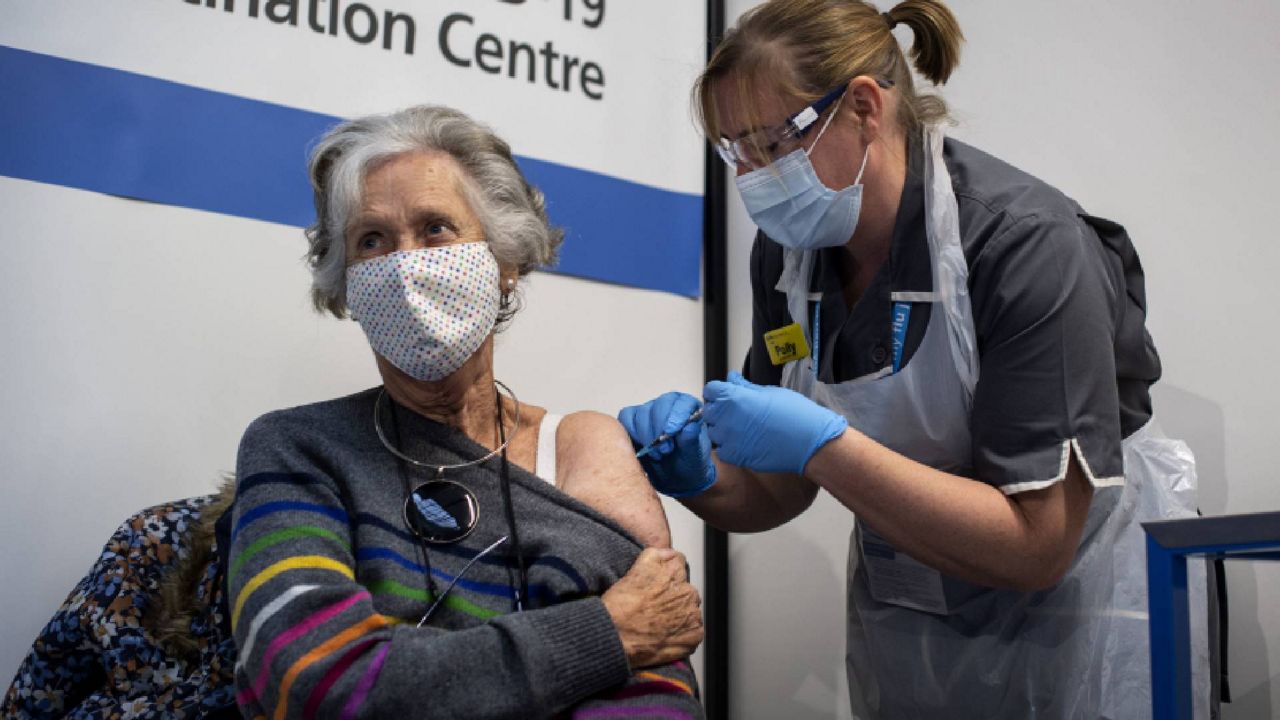
Experts say the coronavirus vaccines currently on the verge of being authorized are generally safe, especially in the short-term. But there are still questions about the vaccines that may not have definitive answers until a large number of Americans are vaccinated, partly because they were developed so quickly.
On Thursday, an independent panel of experts will review Pfizer/BioNTech’s candidate for a COVID-19 vaccine, and the Food and Drug Administration could issue an emergency authorization for the vaccine in the days after. Moderna’s candidate will get a similar review one week after, meaning two vaccines could be approved by the U.S. within a matter of weeks.
Spectrum News spoke with experts across the country to dig deeper on the unanswered questions about the Pfizer and Moderna vaccines.
“Right now, there's no evidence coming out of the study that would indicate whether the vaccine prevents infection,” said Dr. Raphael Viscidi of Johns Hopkins University, who helped develop a vaccine strategy during the first SARS epidemic.
Both Pfizer and Moderna’s trials tested whether their vaccines prevented their participants from getting sick, not whether it prevented them from being infected and potentially passing on the virus to others.
“Would the vaccinated people be similar to that 40 percent of people who have an asymptomatic infection?” Viscidi wondered. “It’s not something they have an answer to right now.”
Experts have said Americans who get vaccinated will still need to practice public health recommendations like wearing a mask and distancing, in order to protect others.
“You don't know the degree to which you're protected. So, you should have some degree of public health measures, obviously, when you get vaccinated,” said Dr. Anthony Fauci in an interview with Spectrum News. “You don't want to abandon them completely and put yourself and your family at risk.”

In an FDA review of Pfizer’s data released Tuesday, the agency also listed transmission of the virus as an unknown.
“Additional evaluations … will be needed to assess the effect of the vaccine in preventing virus shedding and transmission,” the review read.
Still, other experts said it’s possible people who are vaccinated won’t have enough of the virus to infect other people.
“It’s really a numbers game,” said Dr. Paula Cannon, a virologist and Associate Professor of Microbiology at the University of Southern California’s medical school.
“A natural expectation would be that there just wouldn’t be as much virus in your body. The reason you’re asymptomatic is that the virus is struggling to establish itself,” she added. “That may suggest that you are just not infectious.”
On Tuesday, the FDA also affirmed the efficacy of Pfizer’s vaccine at 95 percent and suggested its overall high efficacy may translate to transmission, but more data are needed.
Once someone is vaccinated for COVID-19, their body should naturally develop antibodies that correspond with the virus and ward off future infection, but it’s still not clear how long those antibodies will last.
“The only thing we can tell is what we can learn from natural infection,” said Dr. Maria Elena Bottazzi of Baylor College of Medicine, who helped develop a COVID-19 vaccine currently being tested in India.
According to the Centers for Disease Control and Prevention, cases of reinfection are rare but some have been reported, and studies on the topic are ongoing.
“By the very definition of a question like that, only time will tell,” Dr. Viscidi said. “After you get a vaccine, you produce a lot of antibodies, and they start to go down …. Depending on the vaccine, they can go down more or less rapidly.”
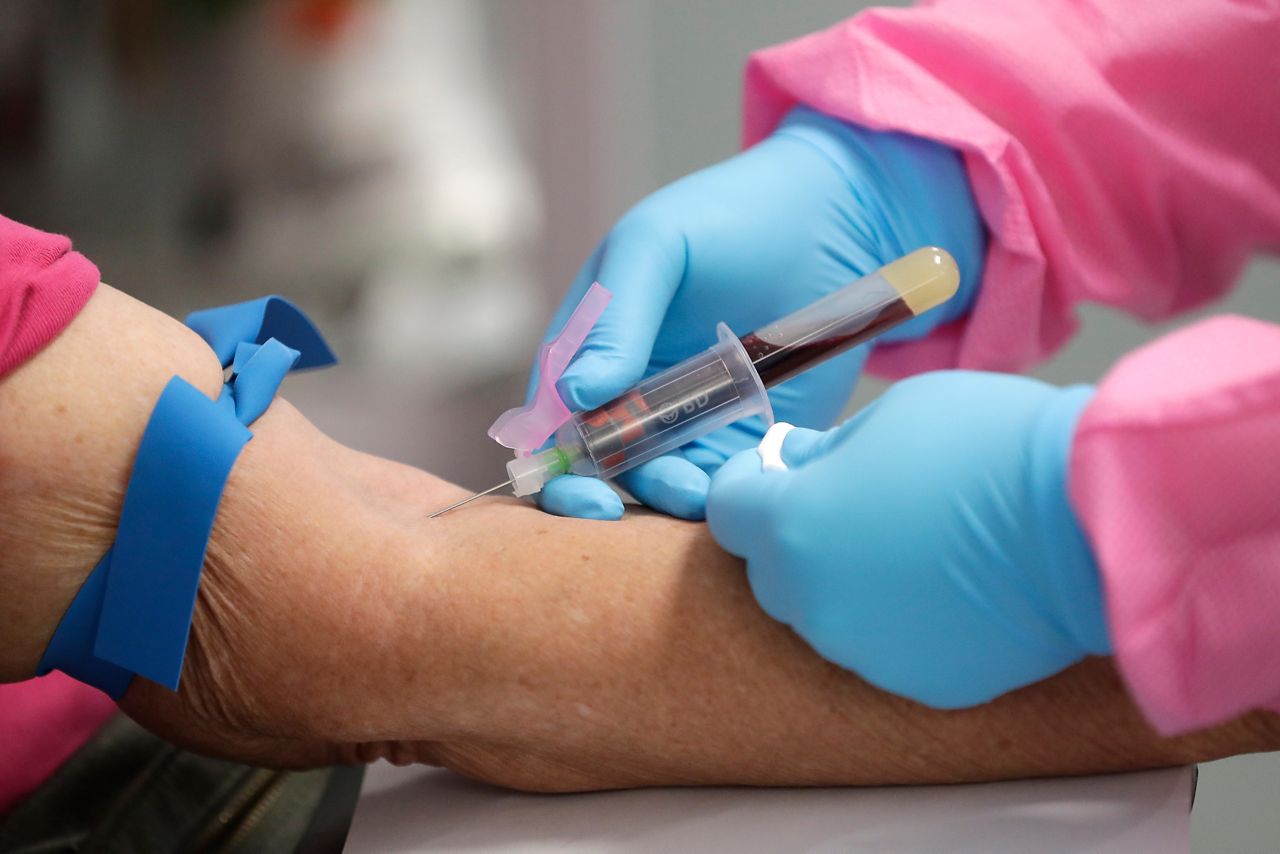
Last week, Moderna published an update on its phase three results showing that participants’ antibodies were still strongly detected 119 days after their first dose. The study — led by the National Institute of Allergy and Infectious Diseases (NIAID) — also found no new side effects from the vaccination, more than three months after volunteers’ first shot.
In its evaluation of Pfizer’s vaccine, regulators wrote that even if they issue an emergency authorization of the vaccine, it will still be considered unapproved. In order to get FDA approval, the agency noted the need for additional studies of “long-term safety and efficacy” including a review of “decreased effectiveness as immunity wanes over time.”