LOS ANGELES — It’s been four months since Jeffrey, who asked that his last name not be used, took part in a clinical trial for an innovate procedure involving two wireless pacemakers.
Unlike traditional wired pacemakers, which are implanted in the skin and use leads that travel through veins and into the heart, the new pill-shaped devices are placed directly in the heart, one in the bottom chamber and one in the top.
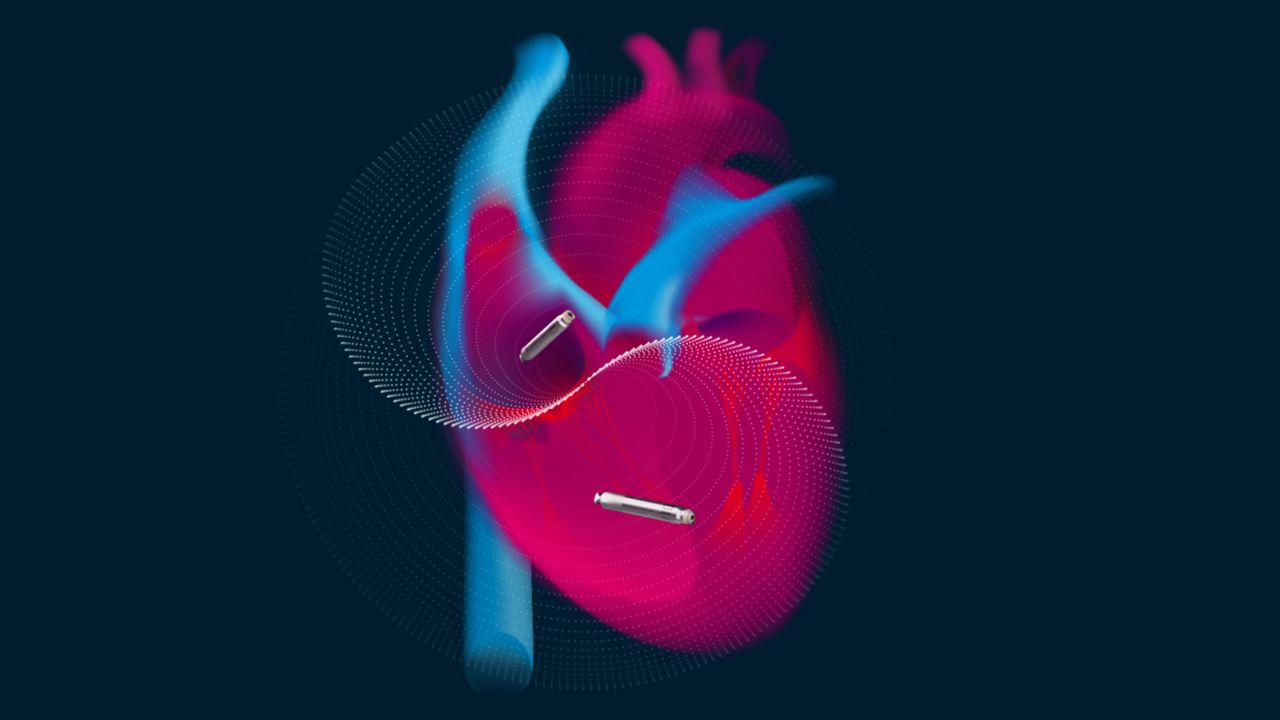
“What’s never been done before is to have two pacemakers in one person and to have them communicate and coordinate their activities with one another,” said Dr. Andrew Zadeh, who’s leading the trial at Keck Medicine of USC.
He was also Jeffrey’s surgeon.
He said the devices communicate through the bloodstream and transmit tiny electrical pulses to keep the heart beating in sync.
“Candidates for a pacemaker implant are any patients who have symptoms related to a low heart rate that’s not enough to give them the blood flow they need,” Zadeh said.
Jeffrey has a rare blood disease called polycythemia vera, which damaged his heart and caused it to beat slowly, but he says he didn’t show any symptoms, only finding out through blood work.
“Most of it was failing while O was sleeping, so I wouldn’t even know,” Jeffrey said.
Because of other health conditions, doctors thought it would be better to avoid a conventional wired pacemaker.
“They can get infected very easily when there’s a bloodstream infection. They also can lead to blockages in the veins,” Zadeh said.
The medical device company Abbott is seeking FDA approval for the dual pacemakers. It launched the study in February and to date, over 300 patients around the world have taken part in the clinical trials, four of the patients at Keck.
“The benefits of the procedure is the fact that everything is implanted directly into the heart. Nothing comes out to the skin where you can feel it,” Zadeh said.
He noted that so far, none of his four patients has reported complications.
Jeffrey was able to go home the next day, but patients are told to avoid heavy lifting for one to two weeks after surgery.
“The wound they used to get in on the groin is super small. I can’t even hardly see it now. It’s tiny and so it’s really non invasive,” Jeffrey said.
While the battery life is still in question, Jeffrey said as a lover of technology, he will take the risk.
“It’s really helped me when I needed it, so I was just glad to have the opportunity,” Jeffrey said.
Let “Inside the Issues” know your thoughts and watch Monday through Friday at 8 and 11 p.m. on Spectrum News 1.



